An interesting article titled “Can platelet-rich fbrin act as a natural carrier for antibiotics delivery? A proof-of-concept study for oral surgical procedures” written by Francesco Bennardo and et. al appears in BMC Oral Health (March 9, 2023, Vol. 23, No. 134). The article seeks to investigate the role of platelet-rich fibrin (PRF) as a carrier for antibiotics delivery for oral surgery.
In the article the authors discuss how mntimicrobial resistance threatens global
health and that judicious prescribing of antibiotics by dental surgeons is needed to help prevent antibiotic resistance. Directly targeting tissues with local drug delivery
strategy has been shown in prior studies as a viable option to reduce unnecessary
antibiotics. In particular autologous platelet concentrates promote tissue healing by releasing autologous growth factors over time and platelet-rich fibrin (PRF) belongs
to a second-generation of this type that did need manipulation after blood collection. PRF has been described by others as a delivery system for antimicrobials. Thus the authors devised a study to evaluate the role of PRF as a local antimicrobial drug delivery system by investigating antibiotic release and antimicrobial activity.
To see if PRF was a natural carrier for antibiotic delivery, PRF was prepared using the leukocyte and PRF protocol. The authors filled various tubes with one tube as control with the others containing increasing amounts of gentamicin (0.25 mg, 0.5 mg, 0.75 mg, and 1 mg), linezolid (0.5 mg, 1 mg, 1.5 mg, and 2 mg), and vancomycin (1.25 mg, 2.5 mg, 3.75 mg, 5 mg). At diferent times, the solution was collected and analyzed. Strains of Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), Streptococcus mitis (S. mitis), Haemophilus influenzae (H. influenzae), Streptococcus pneumoniae (S. pneumoniae), and Streptococcus aureus (S. aureus) were used to assess the antimicrobial effect of the PRF membranes prepared with the antibiotics and those without antibiotics.
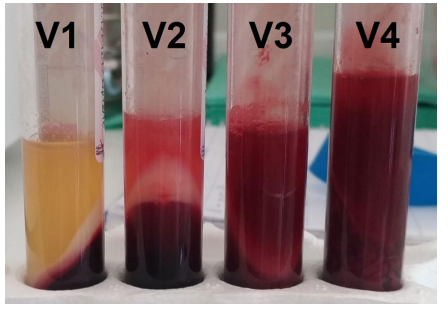
The authors found, vancomycin disrupted PRF formation. Gentamicin and linezolid did not change the physical properties of PRF and were released from membranes in the time intervals studied. The PRF membrane with gentamicin showed significant antibacterial activity against all tested microorganisms. The PRF membrane with linezolid had antibacterial activity against all the tested microorganisms except for E. coli and P. aeruginosa. Further the control PRF showed slight antibacterial activity against all tested microorganisms. The authors state:
“…we documented that PRF could be prepared with antibiotic loading, and the drug is subsequently released from the membrane with an antimicrobial effect.”
From the findings the authors feel that PRF loaded with antibiotics allowed the release of antimicrobial drugs in an efective concentration. It is possible to use PRF loaded with antibiotics after oral surgery to help reduce the risk of post-operative infection and augement systemic antibiotic therapy while preserving the healing properties of PRF. The authors mentioned several limitations of the study which included the lack of analysis of the release of growth factors and a small sample size.
In the future the authors suggest additional studies to prove that PRF loaded with antibiotics represents a topical antibiotic delivery tool for oral surgical procedures. They would also like to evaluate autologous platelet concentrates use as a drug delivery system for antibiotics and other medications through the use of various preparation protocols.
